Vect-Horus has set up a unique VECTrans® technology platform for screening and identification of vectors of different nature from large libraries of molecules to identify efficient vector/receptor systems:
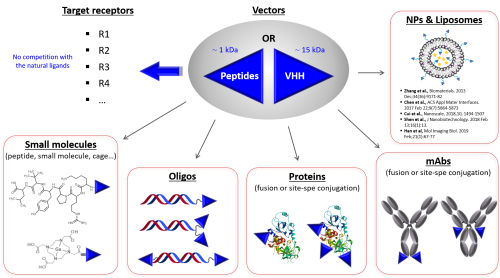
- Peptide vectors: because all-natural and linear peptides display very poor proteolytic resistance, Vect-Horus has developed a deep expertise in medicinal chemistry-based peptide optimization using different techniques including Ala-scan or D-scan, truncations and, most importantly, cyclization and introduction of non-natural amino acids to yield peptide variants with a broad range of biological properties: affinities from the micromolar up to the low nanomolar range and in vitro blood stability up to 10 hours for a 1kDa peptides.
- sdAbs: small antibodies are the smallest (MW < 15 kDa) functional antigen-binding fragments that are derived from heavy chain-only (VHH) camelid antibodies. Different families of sdAbs are currently developed that target different receptors. Using state-of-the-art molecular biology techniques, these VHH vectors are also optimized and diversified to generate variants with distinct and unique features that can be tailored for specific applications.
All Vect-Horus’ vectors bind both non-human and human forms of their target receptor, to ensure efficient translation into the clinics. Furthermore, they are selected for their absence of competition with the main endogenous ligands of the targeted receptors.
Moreover, as opposed to targeting agents such as antibodies, smaller peptide- and small antibody-based vectors are endowed with unique advantages, including high coupling versatility, easy manufacturing, low cost, high tissue penetration potential and low immunogenicity, promoting their use in a wide range of platforms that can be leveraged in specific medical applications.
These peptide- and small antibody-based vectors are protected by several patent families.
Vect-Horus has developed and acquired a deep understanding of the rational design of new vector-payload complex for optimal targeted delivery in a given application. This expertise is key to increase therapeutic or diagnostic potential of new candidates.
All peptide- or small antibody-based vectors developed by Vect-Horus can be conjugated to low molecular weight organic molecules or large protein conjugates in a monovalent or multivalent format, in order to best control and optimize the vector/receptor interaction modalities. Multiple approaches have been developed to generate high quality investigational vector-payload complex. Beyond molecular-scale conjugates, our vectors are also amenable to conjugation to and decoration of any supramolecular delivery vehicle.
Specifically, Vect-Horus has developed a deep in-house expertise in the following fields:
- Protein engineering and vectorization: protein payloads (including antibodies or antibody fragments) can be conjugated to Vect-Horus’ vectors by genetic fusion and subsequently produced in different prokaryote or eucaryote systems to generate quality-controlled conjugates.
- Chemical coupling of Vect-Horus’ vectors to virtually any kind of therapeutic or imaging payloads: Vect-Horus can generate adequately functionalized building blocks for further conjugation using different linkers, either stable or cleavable in specific biological microenvironments.
Each vectorized payloads are delivered as quality-controlled and highly pure batches. Different techniques are used such as Size Exclusion Chromatography (SEC) to yield highly pure and monomeric conjugates, SDS-PAGE gel analysis and MALDI-TOF mass spectrometry as a control of their identity, molecular weight and purity,...
Vect-Horus has already established proof of concept of its VECTrans® technology in animals using molecules of different classes, thereby demonstrating the broad applicability and flexibility of its technology platform.
Small antibody- or peptide-based vectors, used in a monovalent or multivalent format, benefit from fast and efficient tissue penetration as well as controlled vector/receptor interaction modalities. These vectors can also be fused or chemically conjugated using site-specific chemical or enzymatic conjugation at their N-terminus or C-terminus to small organic molecules such as DOTA or NODAGA chelators, siRNAs, peptides, large biomolecules including antibodies, and Lipid NanoParticles (LNPs).
Vector properties can vary depending on the nature, the size of the payload, spacer or linker introduction and the coupling valency. Vect-Horus has developed and acquired a deep understanding of the rational design of new vector-payload complex for optimal targeted delivery. This expertise is key to increase therapeutic or diagnostic potential of new candidates.
Vector-payload complex generated by Vect-Horus are designed to display a dual function, namely i) target a specific cell-surface endocytic receptor expressed by target cells of a given pathophysiological tissue, and ii) deliver the therapeutic or imaging payload in the right subcellular compartment (biophase) in pharmacological/sufficient amounts.
Following engineering and release of high-quality vector-payload complex, Vect-Horus has developed various in vitro models to evaluate their properties and verify that each moiety of the complex retains its properties. Indeed, not only each vector-payload complex should retain the expected biological activity of the payload, but it should also retain full targeting potential. Because securing the transition from preclinical settings to the clinics is of paramount importance, the in vitro screening steps are conducted on rodent, non-rodent and human forms of the target receptor, using both acellular systems such as Surface Plasmon Resonance (SPR) and a variety of cell lines including those engineered by Vect-horus to express the receptors of interest.
Beyond these crucial initial validation steps, additional expertise is often required to better understand and evaluate the fate of the vector-payload complex once endocytosed into brain endothelial cells (ECs) or into any target cell type of the body. Over the years Vect-Horus has thus developed the following expertise:
- In vitro BBB models: Vect-Horus has developed and undergoes continuous optimization of in vitro BBB models based on primary rat endothelial cells that recapitulate the main characteristics of the in vivo BBB, taking into account the neurovascular unit to predict brain exposure of molecules of interest.
- Intracellular trafficking in relevant non-endothelial cellular models : the rational engineering of new vector-payload complex and early investigation of their intracellular trafficking profile in vitro is of paramount importance for Vect-Horus to i) understand the target engagement modalities of the vectors we develop, and ii) allow selection of candidates with optimal intracellular delivery potential in the appropriate subcellular compartment.
Once vector-payload candidates have been screened in vitro to assess their main biological properties, including receptor-mediated targeting and endocytosis in relevant cellular models, the most promising candidates are subsequently evaluated in vivo to assess their potential to trigger efficient and specific targeting and delivery of the payload at the right place and with an adequate exposure.
In vivo evaluation of vector-payload candidates can be performed at Vect-Horus through different modalities and most relevant read-outs, depending on their nature as well as their intended application:
- Pharmacokinetics (PK) and biodistribution in vivo studies: re-engineering of a therapeutic or imaging payload should translate into a modified PK and distribution profile, with a higher exposure in the target tissue/organ. In order to assess the efficiency of our targeted delivery approach, in vivo PK and biodistribution studies are performed using different modalities, including ELISA-based bioanalysis, fluorescence live-imaging, PET imaging…
Vect-Horus has also developed a deep and specific expertise in brain/neuro-PK. Beyond the quantification of the vector-payload in the whole brain, deciphering the extent of exposure in different brain compartments is crucial to guide selection of candidates. Thus, our brain PK studies not only include the quantification of candidates in brain microvessels but, most importantly, also in brain extracellular fluid (ECF) as well as brain parenchyma (i.e. neural cells including neurons, microglial cells, astrocytes…).
- Efficacy in vivo studies: Efficacy studies in relevant animal models (CNS diseases, in particular neurodegenerative diseases, cancer models…) can also be performed to evaluate the benefit obtained from vectorization of a pharmacological agent, using histopathological criteria or behavioral, learning and memory tests.